0.277 mol ammonium acetate Convert to grams: mol NH 4 C 2 H 3 O 2 Step 1: Write the formula NH 4 C 2 H 3 O 2 Step 2: Write the given information. - ppt download

SOLVED:A 7.36-g sample of copper is contaminated with an additional 0.51 g of zinc. Suppose an atomic mass measurement is performed on this sample. What would be the apparent measured atomic mass?

The density of a pure substance A whose atoms are in cubic closed packed arrangement is 1 g/cc. If all the tetrahedral voids are occupied by B atoms, what is the density (

SOLVED:CHAPTER 2 REVIEW WORKSHEET AND KEY The Mole 1) How many zinc (Zn) atoms are contained in 5.16 moles of Zn? How many moles of He are 221,000 He atoms? How many
Solved] 4) A sample of zinc sulfide contains 0.563 g of zinc and 0.276 g of sulfur. How many moles of zinc are there in the sample? How many moles o... | Course Hero

SOLVED:Zinc metal can be obtained from zinc oxide (ZnO) by reacting the oxide with the element carbon. The products of the reaction are Zn and COz. What mass of zinc oxide is

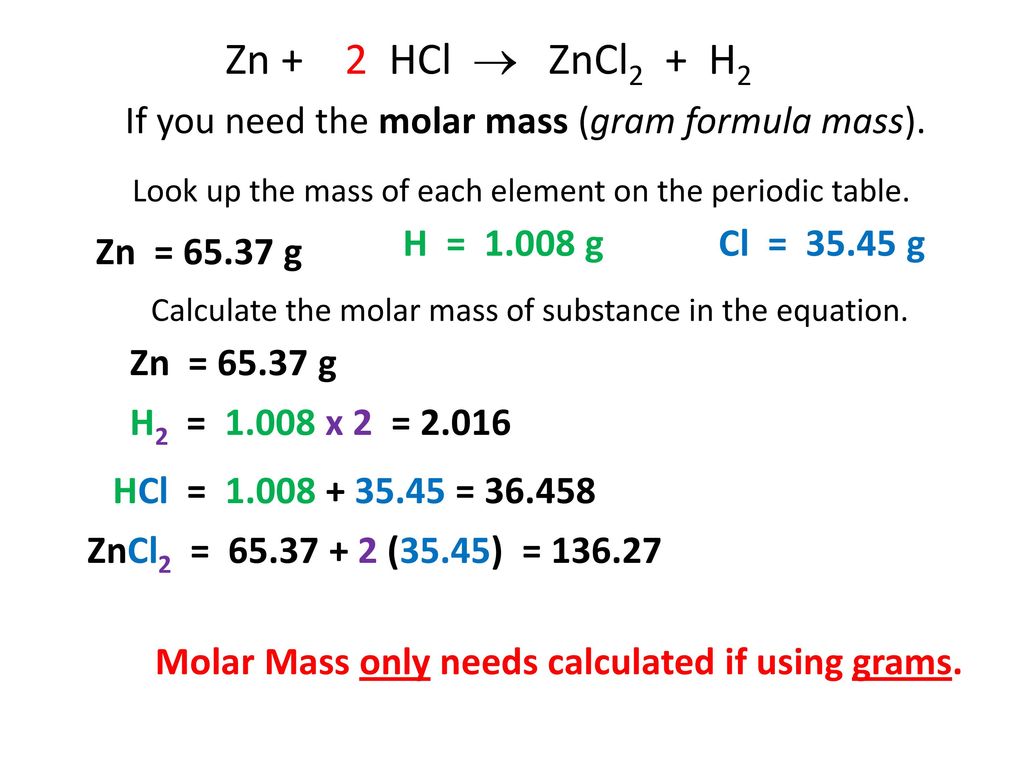
1 General Chemistry CHEM 110 Dr. Nuha Wazzan Chapter 3 Mass Relationships in Chemical Reactions. - ppt download

What mass of zinc can be produced by the electrolysis of zinc sulphate solution when a steady current of 0.015 ampere is passed for 15 minutes ? Given that atomic mass of

The density of a pure substance A whose atoms are in cubic closed packed arrangement is 1 g/cc. If all the tetrahedral voids are occupied by B atoms, what is the density (





/atomic-mass--58dc0d885f9b58468332c41b.jpg)