![Jonathan Weissman on Twitter: "[1/9] Just how many cases of myopericarditis in youngsters during Pfizer's clinical trials is enough for our regulators to intervene and cancel this drug? Here are 3 case Jonathan Weissman on Twitter: "[1/9] Just how many cases of myopericarditis in youngsters during Pfizer's clinical trials is enough for our regulators to intervene and cancel this drug? Here are 3 case](https://pbs.twimg.com/media/FOEowojXoAIZncH.jpg)
Jonathan Weissman on Twitter: "[1/9] Just how many cases of myopericarditis in youngsters during Pfizer's clinical trials is enough for our regulators to intervene and cancel this drug? Here are 3 case

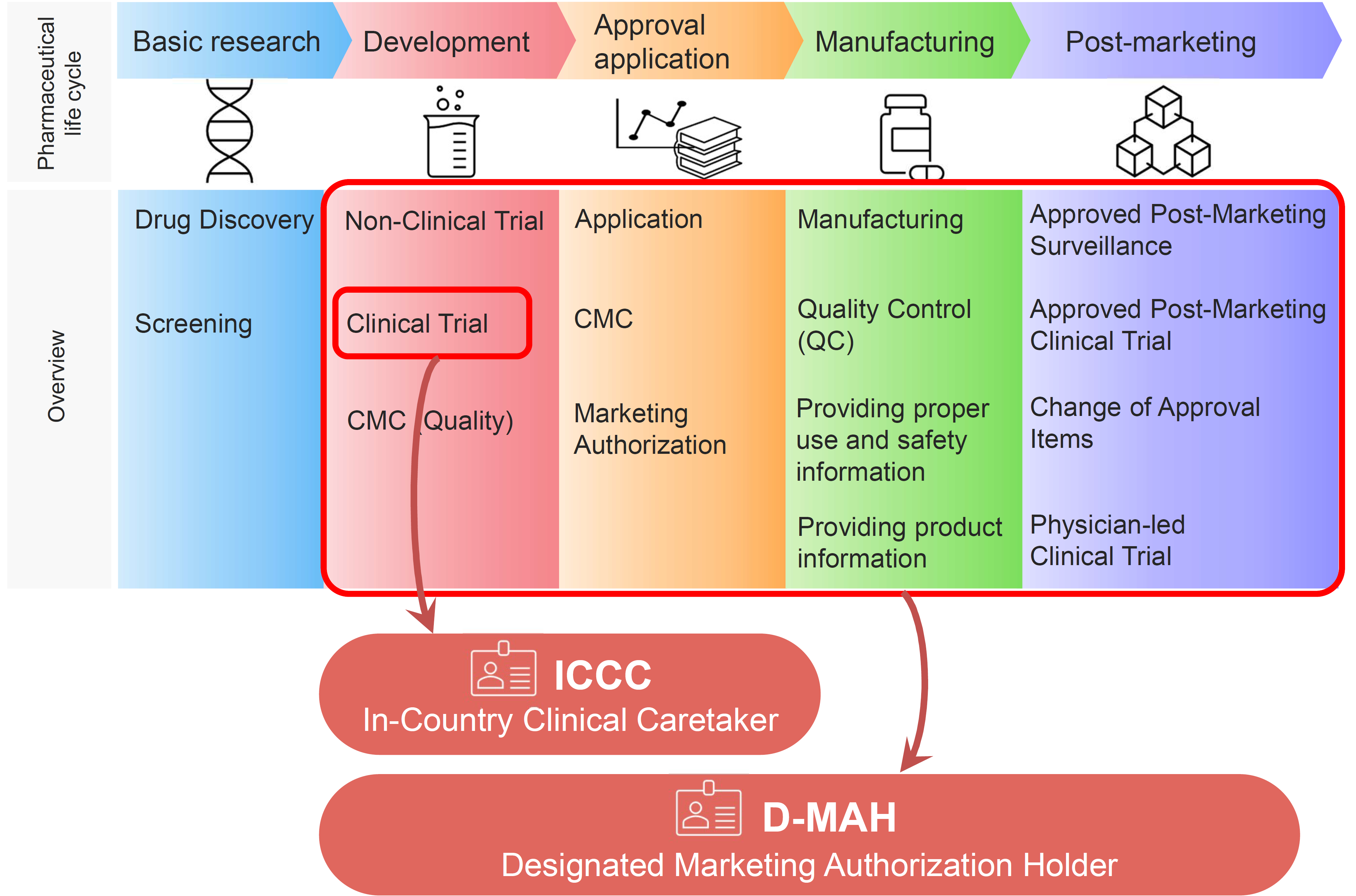
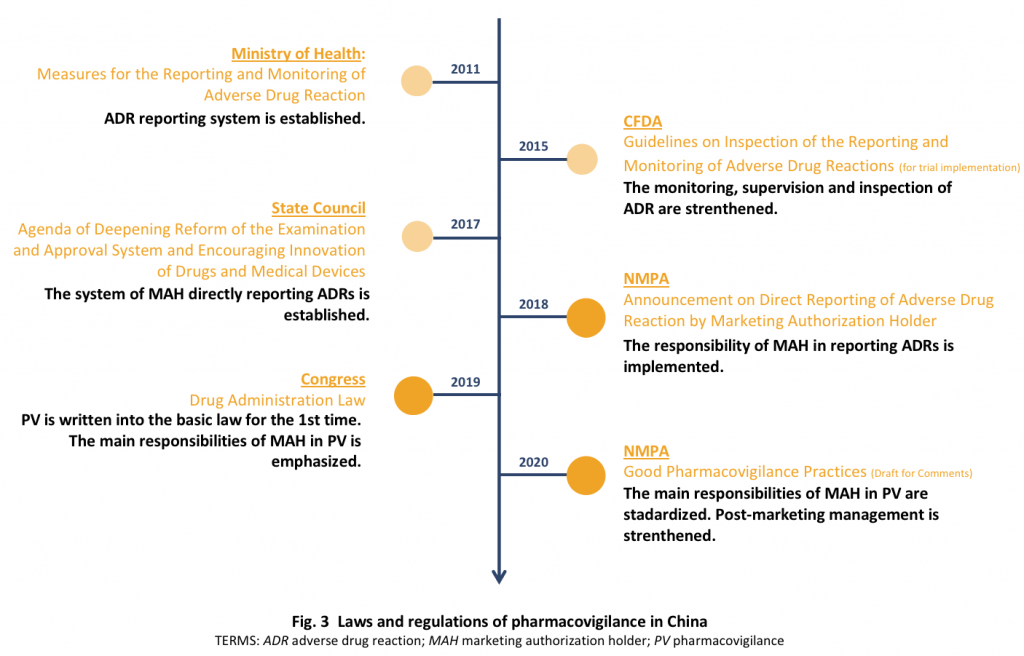
Summary of the signal management process within the European Union and... | Download Scientific Diagram



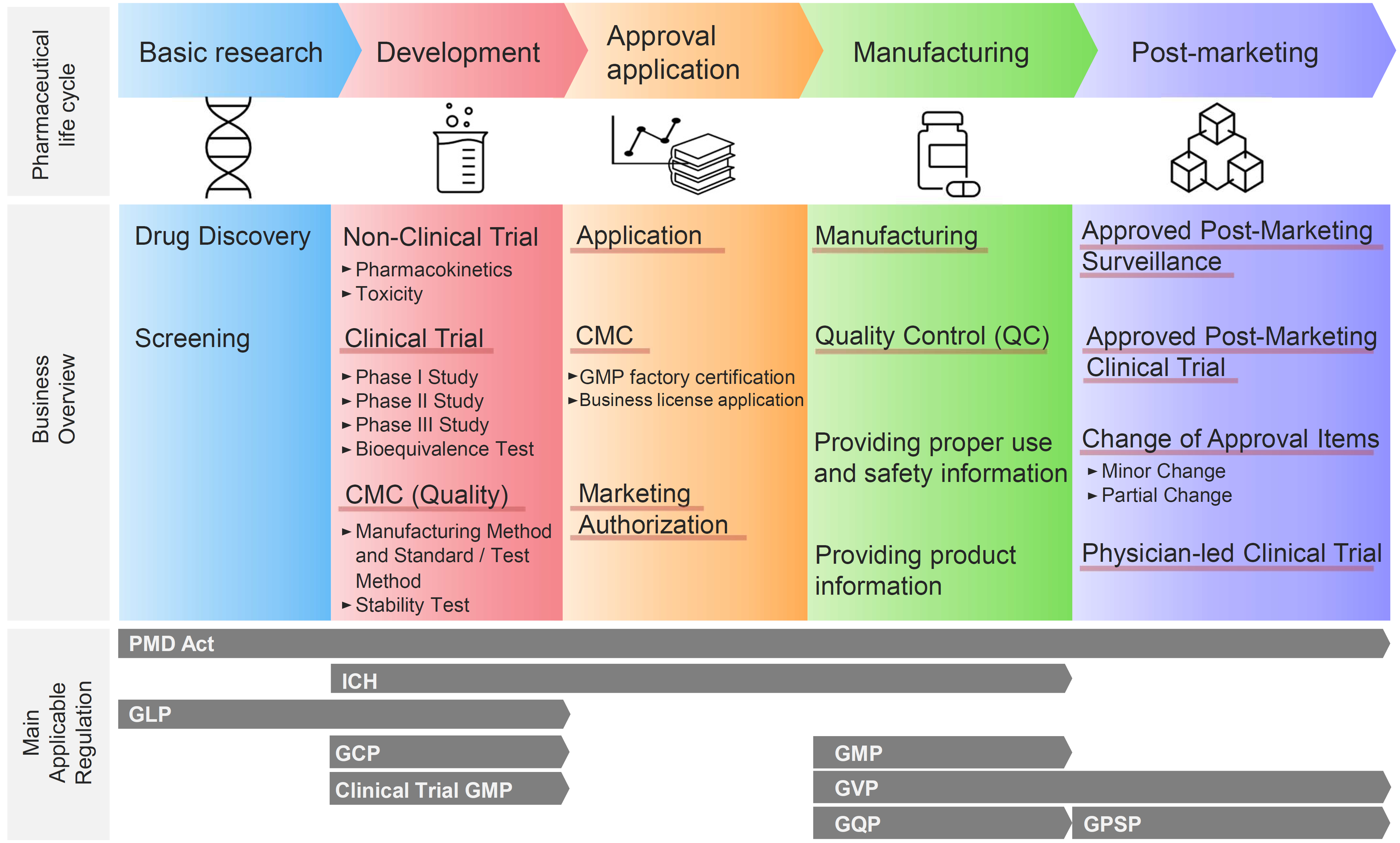





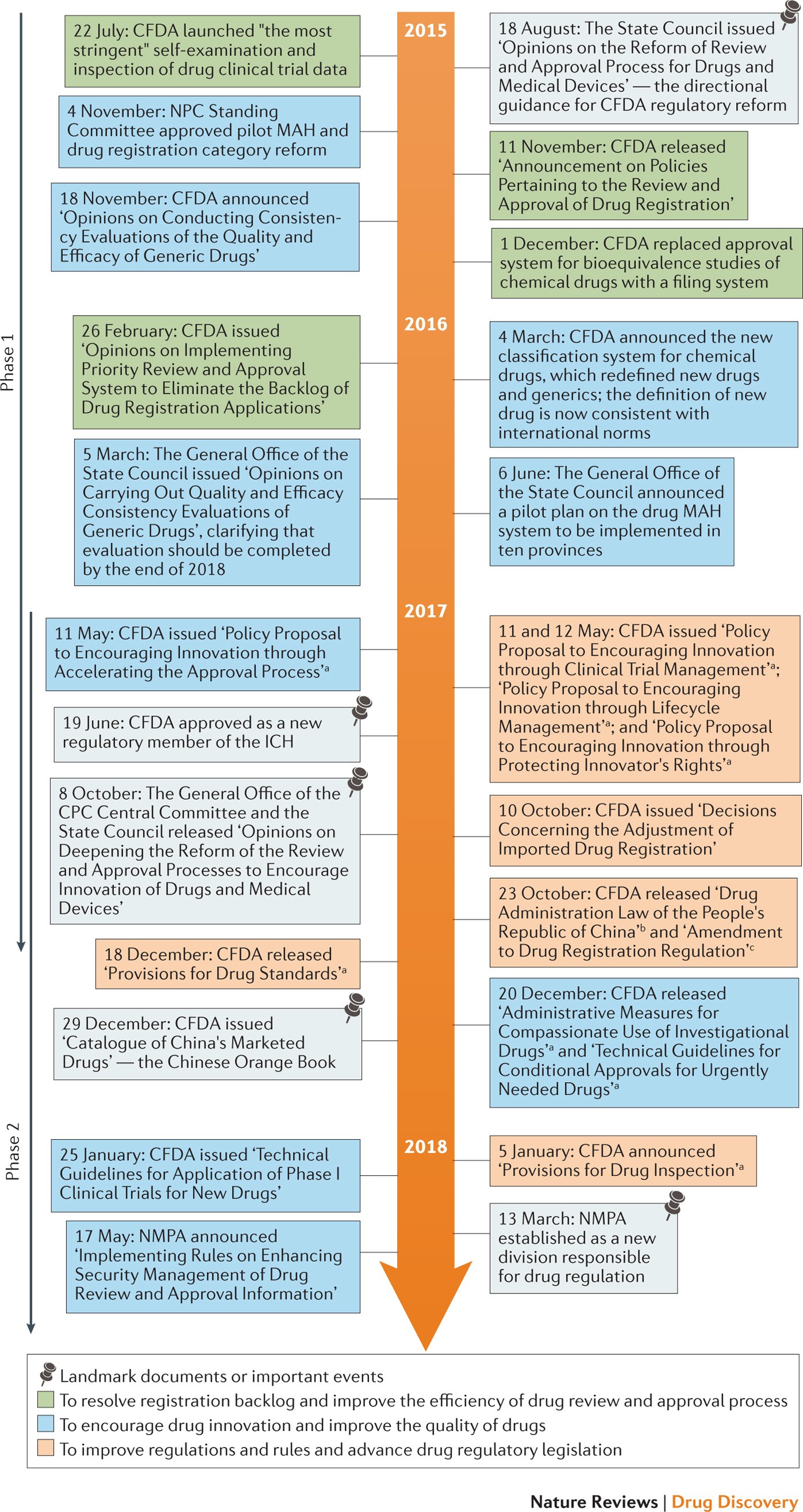
![History of EU regulations applying to PASS [4-6,49]. 1 The 2012 PV... | Download Scientific Diagram History of EU regulations applying to PASS [4-6,49]. 1 The 2012 PV... | Download Scientific Diagram](https://www.researchgate.net/publication/316589487/figure/fig1/AS:613968951382019@1523393013318/History-of-EU-regulations-applying-to-PASS-4-6-49-1-The-2012-PV-legislation-superseded.png)