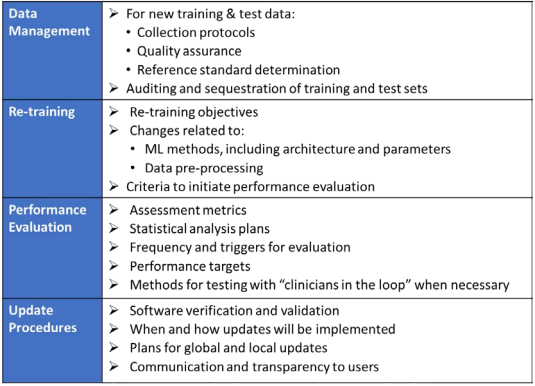
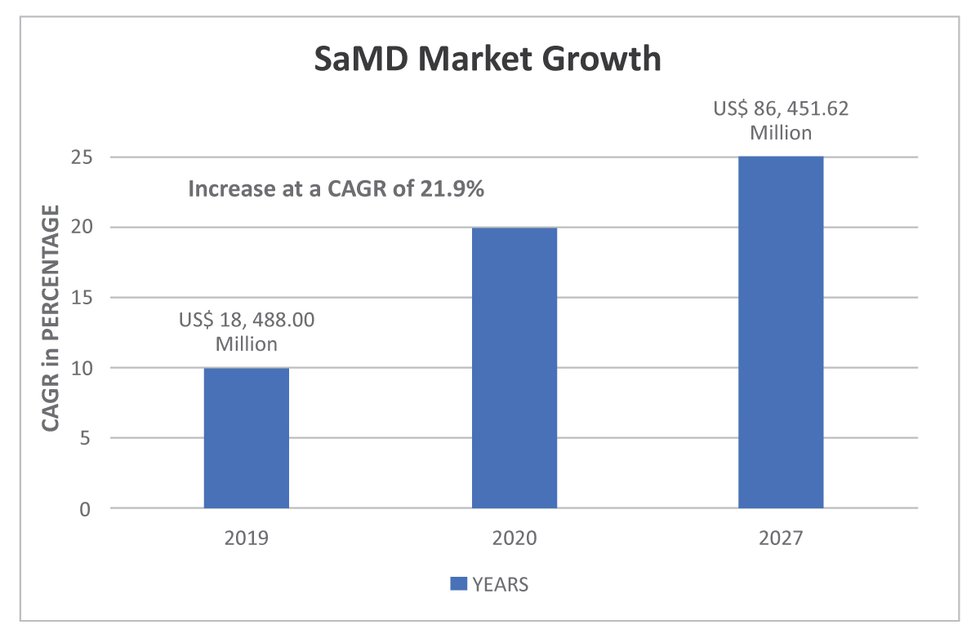
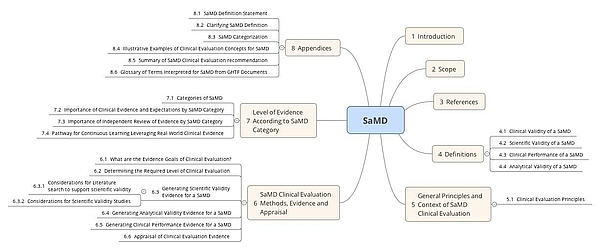
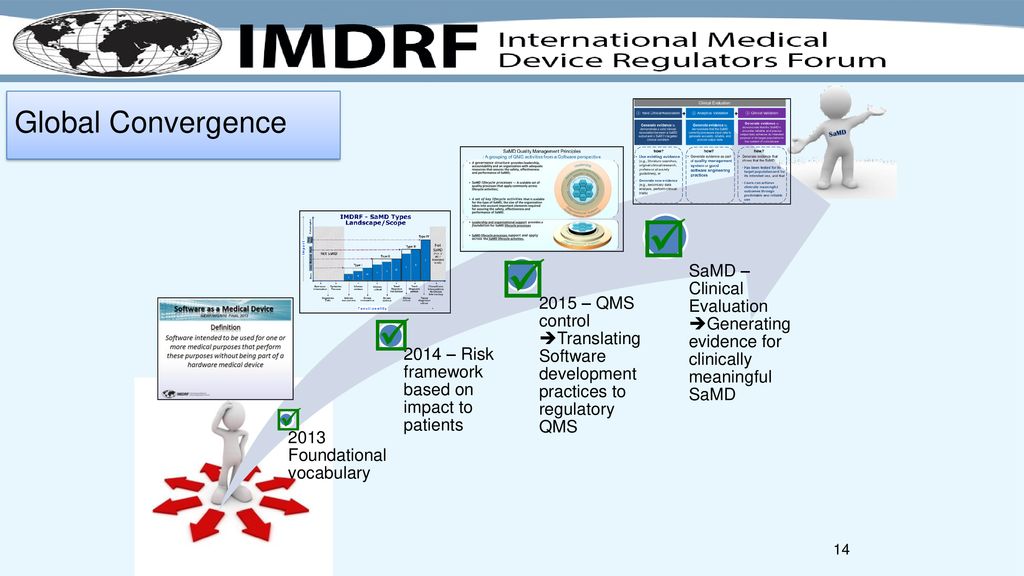
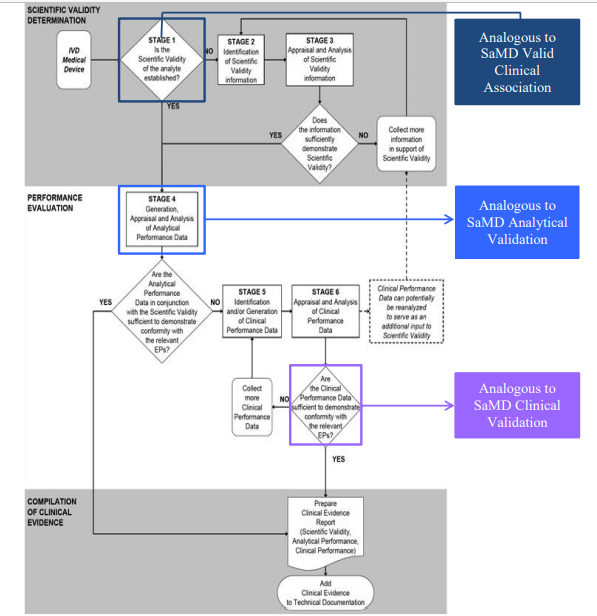
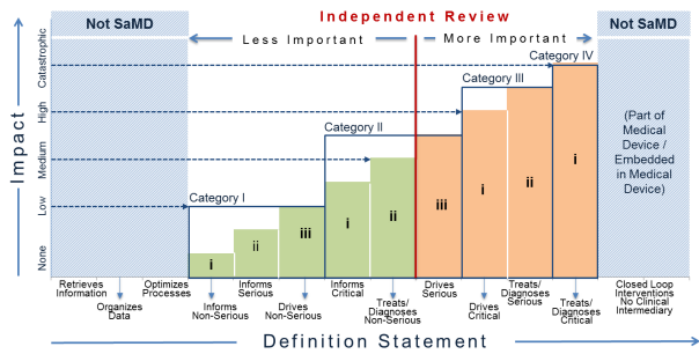
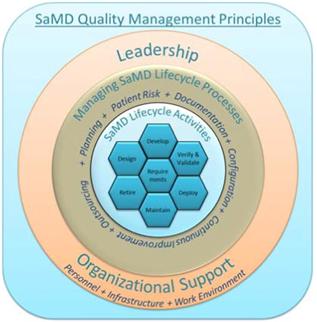
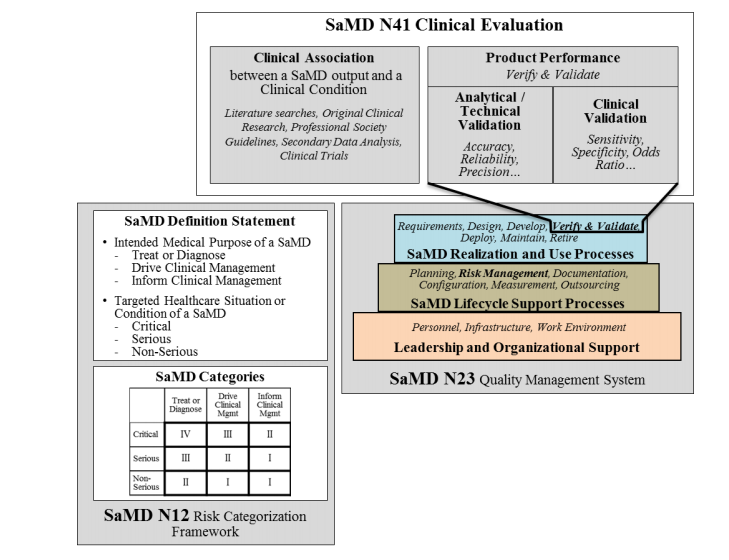
Fillable Online Software as a Medical Device (SAMD): Clinical Evaluation ... Fax Email Print - pdfFiller

Software as a medical device: Here's how the regulatory landscape is changing - Medical Design and Outsourcing

Global Medical Device Podcast: What You Need to Know About Clinical Evaluation & Validation for Software as a Medical Device (SaMD) | Proxima CRO